Trulicity demonstrates recognised characteristics of the GLP-1 receptor agonist class1,2
- Potential for weight loss*
- Comparable rate of hypoglycaemia
-
Transient gastrointestinal adverse reactions
- In clinical trials, the majority of gastrointestinal events were reported during the
first 2 to 3 days after the initial dose and declined with subsequent doses
*Trulicity is not indicated for weight loss, and weight change was a secondary endpoint in clinical trials.
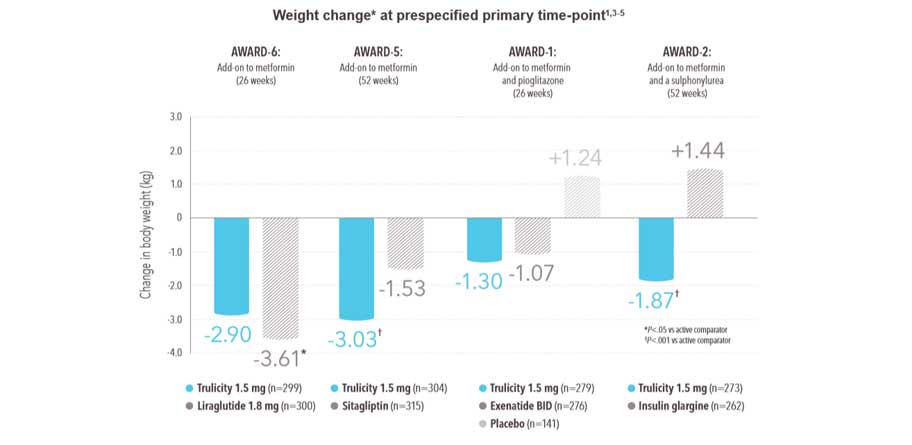
Most patients experienced a secondary benefit of weight loss* with Trulicity 1.5 mg, regardless of concomitant therapy1 :
-
Trulicity 1.5 mg was associated with sustained weight reduction* over the duration of studies (from baseline to final time point -0.35 kg to -2.90 kg)
- In AWARD-6, although both treatment groups experienced weight loss,* the amount of weight loss was significantly greater with liraglutide 1.8 mg
All n values refer to intent-to-treat population.
In AWARD-1, AWARD-2, and AWARD-5, additional groups received Trulicity 0.75 mg, which is the recommended dose for monotherapy. For potentially vulnerable populations, such as patients ≥75 years of age, 0.75 mg once weekly can be considered as a starting dose.
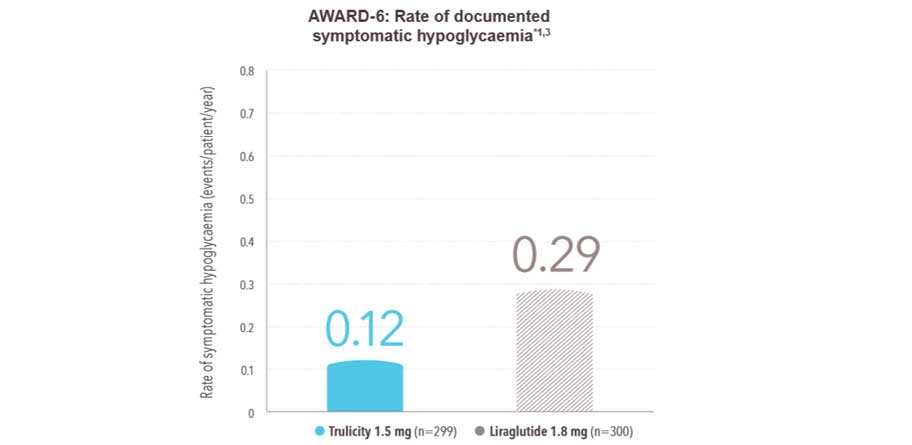
Comparable rate of hypoglycaemia vs liraglutide 1.8 mg1 :
-
The rate of documented symptomatic hypoglycaemia (episodes/patient/year) was 0.12 with Trulicity 1.5 mg and 0.29 with liraglutide 1.8 mg
-
No cases of severe hypoglycaemia were observed
- 26-week active-controlled phase III noninferiority study
- Treatment was added to background therapy with metformin
- Primary endpoint met: noninferiority of Trulicity 1.5 mg vs liraglutide 1.8 mg in HbA1c reduction from baseline to 26 weeks (-1.42% vs -1.36%, P<.001 for noninferiority)
- Data shown are for secondary endpoint
- All n values refer to intent-to-treat population
*Documented symptomatic hypoglycaemia and blood glucose ≤3.9 mmol/L.
Please be aware: Patients receiving Trulicity in combination with a sulphonylurea or prandial insulin may have an increased risk of hypoglycaemia. The risk may be lowered by reducing the dose of sulphonylurea or insulin.
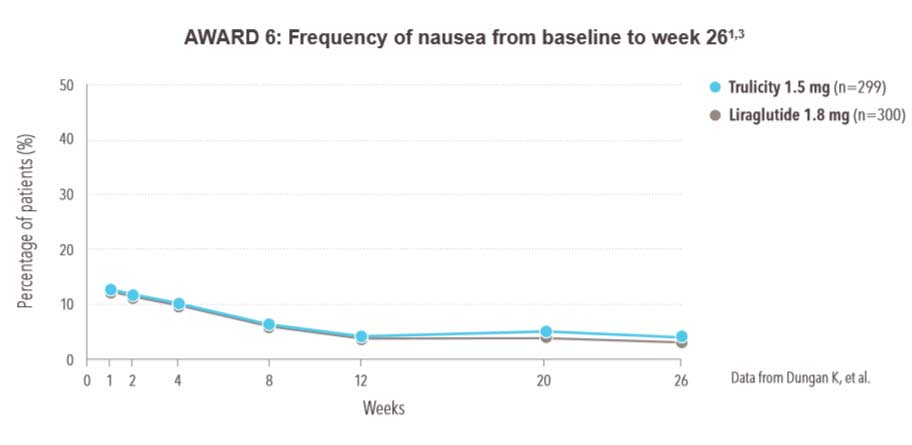
Gastrointestinal side effects were the most common adverse reaction with Trulicity1 :
- Gastrointestinal side effects are typical of the GLP-1 receptor agonist class2
- Gastrointestinal side effects in clinical trials were usually mild to moderate1
- In clinical pharmacology studies, most gastrointestinal side effects occurred during the first 2 to 3 days after the initial dose, and incidence declined with subsequent doses1
- In clinical trials, less than 2% of Trulicity-treated patients withdrew due to nausea1
-
Incidence of nausea was comparable with Trulicity 1.5 mg and liraglutide 1.8 mg3
- 26-week active-controlled phase III noninferiority study
- Treatment was added to background therapy with metformin
- Patients randomised to liraglutide started at 0.6 mg/day in week 1, then were uptitrated to 1.2 mg/day in week 2 and to 1.8 mg/day in week 3
- Primary endpoint met: noninferiority of Trulicity 1.5 mg vs liraglutide 1.8 mg in HbA1c reduction from baseline to 26 weeks (-1.42% vs -1.36%, P<.001 for noninferiority)
- Data shown are for secondary endpoint
- All n values refer to intent-to-treat population
Abbreviated Prescribing Information
Review the abbreviated prescribing information for Trulicity.
References
- Trulicity Summary of Product Characteristics.
- Meier JJ. GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol. 2012;8:728-742.
- Dungan KM, Povedano ST, Forst T, et al. Once-weekly dulaglutide versus once-daily liraglutide in metformin-treated patients with type 2 diabetes (AWARD-6): a randomised, open-label, phase 3, non-inferiority trial. Lancet. 2014;384:1349-1357.
- Nauck M, Weinstock RS, Umpierrez GE, Guerci B, Skrivanek Z, Milicevic Z. Efficacy and safety of dulaglutide versus sitagliptin after 52 weeks in type 2 diabetes in a randomized controlled trial (AWARD-5). Diabetes Care. 2014;37:2149-2158.
- Wysham C, Blevins T, Arakaki R, et al. Efficacy and safety of dulaglutide added onto pioglitazone and metformin versus exenatide in type 2 diabetes in a randomized controlled trial (AWARD-1). Diabetes Care. 2014;37:2159-2167.