Trulicity Pen
Dosing that fits into a busy life:
A ready-to-use pen designed with patients in mind:
Administration that 99% of patients found easy2,3 :
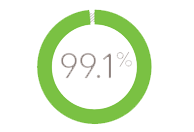
99.1% of 211 injection-naive patients were successfully self-injecting after 3 weeks3 :
Discover how the dulaglutide molecule was designed with the patient in mind.
Abbreviated Prescribing InformationReview the abbreviated prescribing information for Trulicity.