* More than 5000 patients have been enrolled in phase III registration trials
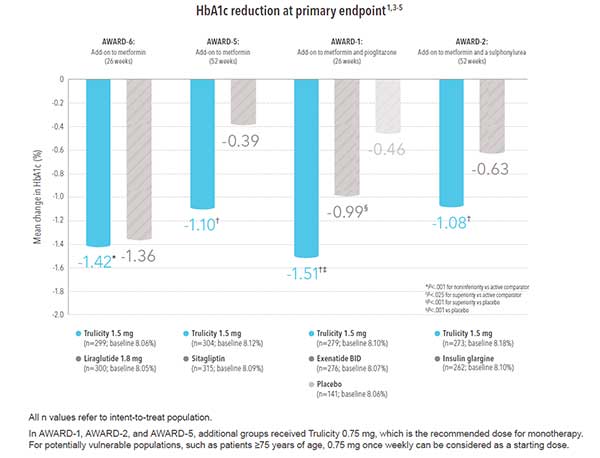
In clinical studies, the range of A1C reduction from baseline was 0.7% to 1.6% for the 0.75 mg dose and 0.8% to 1.6% for the 1.5 mg dose; the percentage of patients achieving A1C <7% ranged from 37% to 69% for 0.75 mg and 53% to 78% for 1.5 mg. 1-5
Comparable glycemic control* to once-daily Victoza® with 85% fewer injections3,9
Once-weekly Trulicity® 1.5 mg provided a 1.42% reduction in A1C compared to a 1.36% reduction for once-daily Victoza 1.8 mg at 26 weeks
 Most common side effects were gastrointestinal (GI). They were nausea, diarrhea, vomiting, and dyspepsia.
Most common side effects were gastrointestinal (GI). They were nausea, diarrhea, vomiting, and dyspepsia.
Once-weekly Trulicity delivered results* across additional clinical trials1,6
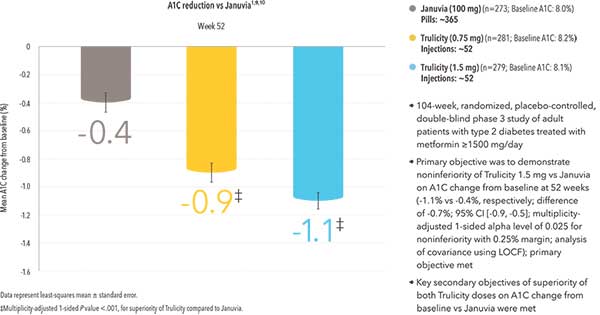
Trulicity demonstrated significant and sustained A1C reduction for up to 2 years* compared to Januvia®12
References
- Trulicity [Summary of Product Characteristics]. Houten, The Netherlands: Eli Lilly and Company; 2014.
- Trulicity Instructions for Use
- Dungan KM, Povedano ST, Forst T, et al. Once-weekly dulaglutide versus once-daily liraglutide in metformin-treated patients with type 2 diabetes (AWARD-6): a randomised, open-label, phase 3, non-inferiority trial [published correction appears in Lancet. 2014;384:1348]. Lancet. 2014;384:1349-1357.
- Umpierrez G, Tofé Povedano S, Pérez Manghi F, et al. Efficacy and safety of dulaglutide monotherapy versus metformin in type 2 diabetes in a randomized controlled trial (AWARD-3). Diabetes Care. 2014;37:2168-2176.
- Giorgino F, Benroubi M, Sun JH, et al. Efficacy and safety of once-weekly dulaglutide versus insulin glargine in patients with type 2 diabetes on metformin and glimepiride (AWARD-2). Diabetes Care. 2015;38(12):2241-2249.
- Blonde L, Jendle J, Gross J, et al. Once-weekly dulaglutide versus bedtime insulin glargine, both in combination with prandial insulin lispro, in patients with type 2 diabetes (AWARD-4): a randomised, open-label, phase 3, non-inferiority study. Lancet. 2015;385:2057-2066.
- Data on file, Lilly USA, LLC. TRU20140919B.
- American Diabetes Association. Standards of medical care in diabetes—2016. Diabetes Care. 2016;39(suppl 1):S1-S112.
- Data on file, Lilly USA, LLC. TRU20150203B.
- Data on file, Lilly USA, LLC. TRU20150203A.
- Wysham C, Blevins T, Arakaki R, et al. Efficacy and safety of dulaglutide added onto pioglitazone and metformin versus exenatide in type 2 diabetes in a randomized controlled trial (AWARD-1) [published correction appears in Diabetes Care. 2014;37:2895]. Diabetes Care.2014;37:2159-2167.
- Nauck M, Weinstock RS, Umpierez G, et al. Efficacy and safety of dulaglutide versus sitagliptin after 52 weeks in type 2 diabetes in a randomized controlled trial (AWARD-5). Diabetes Care. 2014;37(8):2149-2158.
 Most common side effects were gastrointestinal (GI). They were nausea, diarrhea, vomiting, and dyspepsia.
Most common side effects were gastrointestinal (GI). They were nausea, diarrhea, vomiting, and dyspepsia.

